83
UDK: 616.31-002.157.2-055.2:618.36
ETIOPATHOGENESIS, SYMPTOMS, SIGNS, DIAGNOSIS AND PROGNOSIS
TREATMENT OF CHRONIC RECURRENT APHTHOSIS STOMATITIS
Khabibova N.N., Saidova L.A., Akhmedov A.B.
Bukhara State Medical Institute
Recurrent aphthous stomatitis (RA) remains the
most frequent ulcerative disease of the oral mucosa,
manifesting as painful round, shallow ulcers with
well-defined erythematous edges and a yellowish-
gray pseudomembranous center [28]. RAS has a
characteristic prodromal burning sensation that lasts
from 2 to 48 hours before the ulcer appears. It occurs
in healthy individuals and usually localizes on the
mucosa of the cheeks, lips, and tongue. Involvement of
heavily keratinized mucous membranes of the palate
and gums is less common. The attacks of the ulcer
may recur at intervals of months to days, affecting
otherwise healthy individuals. Aphthous ulcers are
usually very painful for the first 4-5 days and may
interfere with eating and speaking during this period.
The first lesions occur in childhood or adolescence,
and it is estimated that up to 25% of the world’s
population is affected by RAS [30,33].
Several factors have been suggested as possible
causative agents of RAS, including local factors, such
as trauma in individuals genetically predisposed to
RAS; microbial factors; nutritional factors, such as
folic acid and B vitamin deficiencies; immunological
factors; psychosocial stress; and allergies to dietary
components [28]. Extensive research has focused
primarily on immunologic factors, but the definitive
etiology of RAS has not yet been established [31].
RAS is subdivided into small (Mirai), large (MaraS),
and herpetiform ulcers (HeraS). More than 85% of
wounds are small ulcers (Mikulic’s aphtha) less than
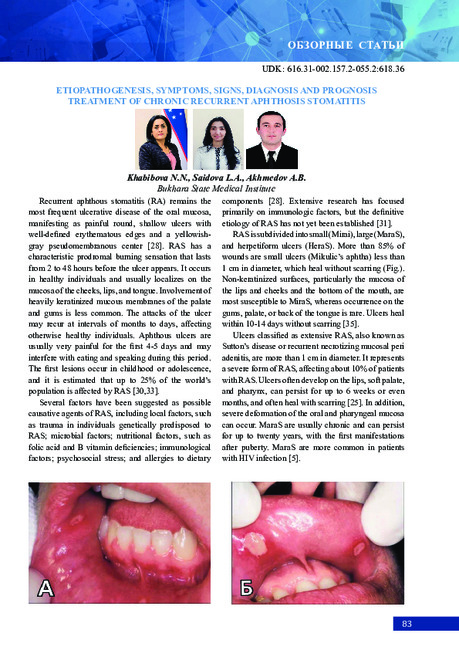
1 cm in diameter, which heal without scarring (Fig.).
Non-keratinized surfaces, particularly the mucosa of
the lips and cheeks and the bottom of the mouth, are
most susceptible to MiraS, whereas occurrence on the
gums, palate, or back of the tongue is rare. Ulcers heal
within 10-14 days without scarring [35].
Ulcers classified as extensive RAS, also known as
Sutton’s disease or recurrent necrotizing mucosal peri
adenitis, are more than 1 cm in diameter. It represents
a severe form of RAS, affecting about 10% of patients
with RAS. Ulcers often develop on the lips, soft palate,
and pharynx, can persist for up to 6 weeks or even
months, and often heal with scarring [25]. In addition,
severe deformation of the oral and pharyngeal mucosa
can occur. MaraS are usually chronic and can persist
for up to twenty years, with the first manifestations
after puberty. MaraS are more common in patients
with HIV infection [5].
ОБЗОРНЫЕ СТАТЬИ

84
Fig.
A small aphthous ulcer on the lower lip (A), a large aphthous ulcer on the upper lip (B), and
a herpetiform form on the lower lip (C). The ulcers have a characteristic erythematous halo and a
yellowish gray pseudomembrane in the center.
Herpetiform ulcers are a rare form of RAS
and occur in about 1.1% of patients with RAS,
clinically different because they appear as
clusters of multiple ulcers scattered throughout
the oral mucosa; despite the name, these lesions
are not associated with herpes simplex virus
[5]. Typically, the characteristic symptoms are
multiple clusters of recurrent small (>5 mm)
painful ulcers that are usually 2-3 mm in size,
although it is not uncommon to see them merge
into larger irregularly shaped ulcers. The lesions
are often located on the floor of the mouth and
on the ventral surface of the tongue. The onset of
HeraS is usually later than in MiraS and MaraS,
and women are reported to be more susceptible
than men.
Epidemiology.
Approximately 20% of the
general population is affected by PAS, but the
incidence ranges from 5% to 50%. This significant
difference in estimated prevalence depends on
the origin of the study groups and populations
as well as study design and methodology.
The presence of aphthous directly at physical
examinations is detected in a smaller percentage
of those examined compared with studies based
on information collected from patient histories.
The second decade of life is considered to be the
peak period for the onset of ASD, with the first
episode in childhood or later in life. The onset
of ASD peaks between the ages of 10 and 19
and becomes less frequent with age, geographic
location, or gender [7,32]. If RAS begins or
increases significantly in severity after the third
decade of life and into adulthood (see Table), this
should reinforce the suspicion that the cause of
the condition may be related to an underlying
disease, such as hematologic or immunologic
abnormality, connective tissue disease, or Behçet
syndrome.
99
Types of recurrent aphthous stomatitis
Form
Small
Big
Herpetiformis
Gender bias
M=F
M=F
M<F
Age of onset
5-19
10-19
20-29
Number of ulcers
1-5
1-10
1-100
Ulcer size (mm)
<10
>10
1-2
Duration (day)
4-14
>30
<30
Frequency of relapses
(months)
1-4
<1
<1
Areas
Lips, cheeks,
tongue, bottom of
the mouth
Lips, cheeks, tongue,
palate, throat
Lips, cheeks,
tongue,
pharynx, palate,
gingiva, bottom
oral cavity
Permanent scarring
No
Continuously
No
Etiopathogenesis.
Many trigger factors
are involved in the etiopathogenesis of ASD:
genetic predisposition, viral and bacterial
infections, food allergies, vitamin and
micronutrient deficiencies, systemic diseases,
increased oxidative stress, hormonal
disorders, mechanical damage. The role of
genetic factors is based on the observation of
families with RAS and has been confirmed
by studies of identical twins; monozygotic
twins have a higher risk of developing the
disease than dizygotic twins [4,38]. No
consistent association has been demonstrated
between specific HLA haplotypes and RAS
[7].
Hematin deficiency.
Hematin (iron, folic
acid, vitamin B12) deficiencies occur in 20%
of patients with RAS [8]; however,
supplementation of missing micronutrients
has been shown to affect the course of the
disease in very few cases [19].
Food allergies.
According to some
researchers, some food ingredients
(chocolate, gluten, cow's milk, nuts),
preservatives, and food colorings can induce
a proinflammatory cascade, and clinical
improvements were observed in some
patients after the introduction of an
elimination diet. However, these findings
have not been confirmed by subsequent
studies [36].
Mechanical Injuries.
In many patients,
lesions may appear shortly after mechanical
irritation of the area. The mechanism of this
reaction remains unknown [23].
Systemic diseases and hormonal
imbalance.
The best-known medical disorder
associated with RAS is Behcet's syndrome.
Recurrent aphthae occur more frequently in
patients with gastrointestinal disorders,
predominantly from the group of chronic
inflammatory bowel diseases [30]. This
correlation may be partly a consequence of
food and micronutrient deficiencies or be
related to autoimmune reactions.
Exacerbation of RAS is observed during the
luteal phase of the menstrual cycle and
during menopause, whereas remission
appears to occur frequently during pregnancy
and in women taking contraceptives [36].
Microbial infections.
The role of many
viruses and bacteria has been emphasized to
support an infectious etiology of RAS.
However, numerous studies have provided
no evidence. Oral streptococcus colonizes
aphthous ulcers, and it has been suggested
that it may cross-react with mitochondrial
proteins, causing damage to the oral mucosa

85
ОБЗОРНЫЕ СТАТЬИ
99
Types of recurrent aphthous stomatitis
Form
Small
Big
Herpetiformis
Gender bias
M=F
M=F
M<F
Age of onset
5-19
10-19
20-29
Number of ulcers
1-5
1-10
1-100
Ulcer size (mm)
<10
>10
1-2
Duration (day)
4-14
>30
<30
Frequency of relapses
(months)
1-4
<1
<1
Areas
Lips, cheeks,
tongue, bottom of
the mouth
Lips, cheeks, tongue,
palate, throat
Lips, cheeks,
tongue,
pharynx, palate,
gingiva, bottom
oral cavity
Permanent scarring
No
Continuously
No
Etiopathogenesis.
Many trigger factors
are involved in the etiopathogenesis of ASD:
genetic predisposition, viral and bacterial
infections, food allergies, vitamin and
micronutrient deficiencies, systemic diseases,
increased oxidative stress, hormonal
disorders, mechanical damage. The role of
genetic factors is based on the observation of
families with RAS and has been confirmed
by studies of identical twins; monozygotic
twins have a higher risk of developing the
disease than dizygotic twins [4,38]. No
consistent association has been demonstrated
between specific HLA haplotypes and RAS
[7].
Hematin deficiency.
Hematin (iron, folic
acid, vitamin B12) deficiencies occur in 20%
of patients with RAS [8]; however,
supplementation of missing micronutrients
has been shown to affect the course of the
disease in very few cases [19].
Food allergies.
According to some
researchers, some food ingredients
(chocolate, gluten, cow's milk, nuts),
preservatives, and food colorings can induce
a proinflammatory cascade, and clinical
improvements were observed in some
patients after the introduction of an
elimination diet. However, these findings
have not been confirmed by subsequent
studies [36].
Mechanical Injuries.
In many patients,
lesions may appear shortly after mechanical
irritation of the area. The mechanism of this
reaction remains unknown [23].
Systemic diseases and hormonal
imbalance.
The best-known medical disorder
associated with RAS is Behcet's syndrome.
Recurrent aphthae occur more frequently in
patients with gastrointestinal disorders,
predominantly from the group of chronic
inflammatory bowel diseases [30]. This
correlation may be partly a consequence of
food and micronutrient deficiencies or be
related to autoimmune reactions.
Exacerbation of RAS is observed during the
luteal phase of the menstrual cycle and
during menopause, whereas remission
appears to occur frequently during pregnancy
and in women taking contraceptives [36].
Microbial infections.
The role of many
viruses and bacteria has been emphasized to
support an infectious etiology of RAS.
However, numerous studies have provided
no evidence. Oral streptococcus colonizes
aphthous ulcers, and it has been suggested
that it may cross-react with mitochondrial
proteins, causing damage to the oral mucosa
Etiopathogenesis.
Many trigger factors are
involved in the etiopathogenesis of ASD: genetic
predisposition, viral and bacterial infections, food
allergies, vitamin and micronutrient deficiencies,
systemic diseases, increased oxidative stress,
hormonal disorders, mechanical damage. The role of
genetic factors is based on the observation of families
with RAS and has been confirmed by studies of
identical twins; monozygotic twins have a higher risk
of developing the disease than dizygotic twins [4,38].
No consistent association has been demonstrated
between specific HLA haplotypes and RAS [7].
Hematin deficiency.
Hematin (iron, folic acid,
vitamin B12) deficiencies occur in 20% of patients
with RAS [8]; however, supplementation of missing
micronutrients has been shown to affect the course of
the disease in very few cases [19].
Food allergies.
According to some researchers,
some food ingredients (chocolate, gluten, cow’s milk,
nuts), preservatives, and food colorings can induce a
proinflammatory cascade, and clinical improvements
were observed in some patients after the introduction
of an elimination diet. However, these findings have
not been confirmed by subsequent studies [36].
Mechanical Injuries.
In many patients, lesions
may appear shortly after mechanical irritation of
the area. The mechanism of this reaction remains
unknown [23].
Systemic diseases and hormonal imbalance.
The best-known medical disorder associated with
RAS is Behcet’s syndrome. Recurrent aphthae occur
more frequently in patients with gastrointestinal
disorders, predominantly from the group of chronic
inflammatory bowel diseases [30]. This correlation
may be partly a consequence of food and micronutrient
deficiencies or be related to autoimmune reactions.
Exacerbation of RAS is observed during the luteal
phase of the menstrual cycle and during menopause,
whereas remission appears to occur frequently during
pregnancy and in women taking contraceptives [36].
Microbial infections.
The role of many viruses and
bacteria has been emphasized to support an infectious
etiology of RAS. However, numerous studies have
provided no evidence. Oral streptococcus colonizes
aphthous ulcers, and it has been suggested that it
may cross-react with mitochondrial proteins, causing
damage to the oral mucosa [29]. Meta-analysis
supports a link between RAS and Helicobacter pylori
infection, but the presence of the bacteria in RAS foci
is controversial.
Stress.
Stressful events are thought to exacerbate
RAS, affect its duration, or cause the onset of disease
[30].
Mucosal fnd Salivary Microbiota.
It is
estimated that the human oral cavity is colonized by
approximately 700 different major bacterial species,
which produce a huge number of different peptides
and polysaccharides of molecular type associated
with pathogens that can interact with each other
and the host immune system to maintain a stable
symbiotic microenvironment during health [27]. If
this balance is disrupted, the symbiotic relationship
shifts, allowing potentially pathogenic species to
colonize or overgrow, causing a pathogenic process
leading to symptoms associated with various diseases
[10]. In general, nine major types of bacteria reside in
the mouth of a healthy person [8]. At the level of the
genus Streptococcus is known to be the most common
genus.
Clinical manifestation and pathogenesis.
Patients with RAS usually experience prodromal
burning sensations that last from 2 to 48 hours
before an ulcer appears. The ulcers are round, with
well-defined erythematous margins and a shallow,
ulcerated center, covered with a yellowish-gray
fibrinous pseudomembrane. Ulcers usually developed
on the non-keratinized oral mucosa, with cheek and
lip mucosa being the most common sites, lasting
approximately 10 to 14 days without scarring (see Table
1). Oral ulcers seen in Behcet’s disease are clinically
similar, but more often are large aphthae [25]. The
microscopic characteristics of RAS are nonspecific.
The pre inflammatory lesion shows subepithelial
inflammatory mononuclear with abundant mast
cells, connective tissue edema, and edge lined with
neutrophils [37]. Epithelial damage usually starts in the
basal layer and spreads through the superficial layers,
eventually leading to ulceration and a superficial
exudate. The presence of extravascular erythrocytes
around the ulcer margin, subepithelial extravascular

86
neutrophils, numerous macrophages loaded with
phagolysosomes, and nonspecific binding of spiny
layer cells to immunoglobulins and complement may
result from vascular seepage and passive diffusion
of serum proteins. These findings suggest that the
pathogenesis of RAS may be mediated by immune
complex vasculitis [16]. The onset of RAS lesions
is associated with a cell-mediated immune response,
T-cell formation and TNF-a production. It has been
given that mononuclear cells in the peripheral blood
of patients with RAS secrete large amounts of TNF-α,
an indication that, TNF-a plays a key role in the
pathogenesis of RAS [17,21,22]. Consequently, TNF-
a-mediated endothelial cell adhesion and neutrophil
chemotaxis initiate the cascade of inflammatory
processes leading to ulceration [3]. Most TNF-α is
produced in response to activation of toll-like receptors
(TLRs), a set of functional membrane receptors
associated with immune response and epithelial
barrier protection. TLRs have both pro-inflammatory
and anti-inflammatory properties. Considering that in
some patients proinflammatory TLRs have been found
to be significantly increased in the epithelium and
own lamina of RAS lesions [26] decreased expression
levels of TLRs with anti-inflammatory activity have
also been found in another group of patients with RAS
[11]. Thus, the role of TLR in the pathogenesis of RAS
still needs to be better defined, but it is possible that an
imbalance of pro-inflammatory and anti-inflammatory
TLR activity may increase susceptibility to RAS in
some individuals.
Therapy.
Therapeutic goals include reducing
ulcer pain, accelerating ulcer healing, and preventing
recurrence [4,9,13,23]. Local therapy and anesthetics
such as lidocaine and benzocaine are used for
short-term pain relief, especially in large ulcers.
Corticosteroids are often used to accelerate ulcer
healing and reduce RAS symptoms. High potency
steroids (dexamethasone, triamcinolone, fluocinonide,
and clobetasol) in mouthwashes are preferred.
Although there is no evidence of a bacterial origin of
RAS, topical antimicrobials such as chlorhexidine,
tetracycline, and diluted hydrogen peroxide have
been associated with accelerated healing of RAS
ulcers. Drugs with antimicrobial, anti-inflammatory,
and analgesic effects have been shown to cause some
positive effects when used as a mouthwash. Of
secondary importance are coating agents that protect
and strengthen the natural mucosal barrier: sucralfate,
bismuth subsalicylate, and oral bioadhesives.
Particularly severe cases of RAS are treated with
systemic therapy: systemic steroids, colchicine,
thalidomide:
- Systemic steroids: a short course. Systemic
steroids can sometimes be used to treat a particularly
severe episode of extensive RA;
- Colchicine: at a dose of 0.6-1.2 mg/day, has
shown encouraging results in reducing the number
and duration of aphthous lesions;
- Thalidomide: controlled trials have demonstrated
thalidomide to be effective in treating RA, causing
complete remission or significant improvement in
most patients.
The use of lasers (CO2, ND: YAG, diode laser)
to relieve symptoms and accelerate RA healing is a
therapeutic option.
References
1.
Акбаров А.Н., Джумаев А. Гигиеническое
состояние протезов у больных с частично
съемными зубными протезами //
Pal
Arch по
археологии Египта/египтологии. – 2020. – Vol. 17,
№6. – Р. 14351-14357.
2.
Алимова Н.П., Асадова Н.Х. Изучение
анатомии через проблемное обучение среди
студентов медиков // Современное состояние
медицинского
образования:
проблемы
и
перспективы: Сб. материалов междунар. учеб.
онлайн-конф. – 2020. – С. 138-139.
3.
Жумаев
А.Х. Method for assessing the state
of the oral mucosa in dental defects // Мед. журн.
Узбекистана. – 2021. – №2.
4.
Хабибова Н.Н., Саидова Л.А., Саидов
А.А. особенности течения рецидивирующего
афтозного стоматита у женщин фертильного
возраста принимающих метотрексат // Тиббиётда
янги кун. – 2022. – Vol. 3 (41). –
P
435-439.
5.
Akintoye S.O., Greenberg M.S. Recurrent
aphthous stomatitis // Dent. Clin. North Amer. – 2005.
– Vol. 49. – P. 31-47.
6.
Akintoye S.O., Greenberger S. Recurrent
Aphthous Stomatitis // Dent. Clin. North Amer. –
2005. – Vol. 49. – P. 31-47.
7.
Albanidou-farmaki E., Deligiannidis A.,
Markopoulos A.K. et al. HLA haplotypes in recurrent
aphthous stomatitis: a mode of inheritance? // Int. J.
Immunol. Genet. – 2008. – Vol. 35. – P. 427-432.
8.
Bik E.M., Long C.D., Armitage G.C. et al.
Bacterial diversity in the oral cavity of 10 healthy
individuals // ISME J. – 2010. – Vol. 4. – P. 962-974.
9.
Cui R.Z., Bruce A.J., Rogers R.S. Recurrent
aphthous stomatitis // Clin. Dermatol. – 2016. – Vol.
34. – P. 475-481.
10.
Doktor M.J., Paster B.J., Abramowicz S. et
al. Alterations in diversity of the oral microbiome
in pediatric bowel disease // Inflamm. Bowel Dis. –
2012. – Vol. 61. – P. 935-942.

87
ОБЗОРНЫЕ СТАТЬИ
11.
Durdiev J.I. Influence of the quality of life
on the formation of the upper jaw in children with
pathologies of the respiratory system // Wld Med. Jl.
Pol. – 2021. – P. 182-186.
12.
Gafforov S.A., Aliev N.H. Improvement of
diagnostic methods and treatment of parafunction
of chewable Muscles in pain syndromes of a High-
Lower jaund joint // J. Adv. Res. Dynam. Contr. Syst.
– 202. – Vol. 12. – P. 2102-2110.
13.
Gafforov S.A., Aliev N.H. Improving the
methods for the diagnosis of nonarticular pathology
of the temporomandibular joint // J. Crit. Rev. – 2020.
– Vol. 7, Issue 18. – P. 875-880.
14.
Gallo А., Barros F., Sugaya N. et al.
Differential expression of toll-like receptor mRNAs in
recurrent aphthous ulceration // J. Oral Pathol. Med. –
2012. – Vol. 41, №1. – Р. 80-85.
15.
Giannetti L., Murri dello Diago A., Lo Muzio
L. Recurrent aphthous stomatitis // Minerva Stomatol.
– 2018. – Vol. 67. – P. 125-128.
16.
Jurge S., Kuffer R., Scully C. et al. Mucosal
disease series. Number VI. Recurrent aphthous
stomatitis // Oral Dis. – 2006. – Vol. 12, №1. – P. 1-21.
17.
Khabibova N.N., Saidova L.A., Saidov A.A.
Improvement of the treatment regimen for recurrent
aphthous stomatitis // Brit. Med. J. – 202. – Vol. 2,
№2.
18.
Khan N.F., Saeed M., Chaudhary S., Khan
N.F. Hematological parameters and recurrent aphthous
stomatitis // J. Coll. Physic. Surg. Pak. – 2013. – Vol.
23. – P. 124-127.
19.
Lalla R.V., Choquette L.E., Fein R.S. et
al. Multivitamin therapy for recurrent aphthous
stomatitis: a randomized, double-masked, placebo-
controlled trial // J. Amer. Dent. Assoc. – 2012. – Vol.
7. – P. 37037.
20.
Landova H., Danek Z., Gaidziok J. et al. Oral
Mucosa and therapy of recurrent aphthous stomatitis //
Ces. Slov. Farm. – 2013. – Vol. 62. – P. 12-18.
21.
Lewkowicz N., Kur B., Kurnatowska A. et
al. Expression of Th1/Th2/Th3/ Th17-related genes
in recurrent aphthous ulcers // Arch. Immunol. Ther/
Exp. (Warsz). – 2011. – Vol. 59, №5. – P. 399-406.
22.
Lewkowicz N., Lewkowicz P., Dzitko K. et
al. Dysfunction of CD41CD25 high T regulatory cells
in patients with recurrent aphthous stomatitis // J. Oral
Pathol. Med. – 2008. – Vol. 37, №8. – P. 454-561.
23.
Natah S.S., Konttineen Y.T., Enattah N.S.
Recurrent aphthous ulcers today: a review of growing
knowledge // Int. J. Oral Maxillofac. Surg. – 2004. –
Vol. 33. – P. 221-234.
24.
Nusratov U.G., Matrizayev L.Yu. Improving
the Quality and Effectiveness of Treatment of Patients
with Dental Anomalies // Eurasian Sci. Herald Open
Acces. Peer Rev. J. – ГОД. – Vol. . – P. 165-169.
25.
Oh S.H., Han E.C., Lee J.H. et al. Comparison
of the clinical features of recurrent aphthous stomatitis
and Behcet’s disease // Clin. Exp. Dermatol – 2009/ –
Vol. 34. №6. – P. e208-e212.
26.
Olimov S.Sh., Bakaev J.N., Safarova M.J.
Aspects of the formation of pain syndrome in the
area of the masticatory muscles in the disease of the
maxillary-mandibular composition // Int. J. Hum.
Comp. Studies. – 2021. – Vol. 3, Issue 1. – Р. 117-121.
27.
Paster B.J., Boches S.G., Galvin J.L. et al.
Bacterial diversity in Human subgingival Plaque // J.
Bacteriol. – 2001. – Vol. 183. – P. 3770-3783.
28.
Porter S.R., Scully C., Pedersen A. Recurrent
aphthous stomatitis // Crit. Rev. Oral Biol. Med. –
1998. – Vol. 9, №3. – P. 306-321.
29.
Riggio M.P., Lennon A., Ghodrathnama F.,
Wray D. Lack of association between Streptococcus
oralis and recurrent aphthous stomatitis // J. Oral
Pathol. Med. – 2000. – Vol. 29. – P. 26-32.
30.
Roger R.S. Recurrent aphthous stomatitis.
Clinical Characteristic and Associated Systemic
Disorders // Sem. Cut .Med. Surg. – 1997. – Vol. 16.
– P. 278-283.
31.
Saidova L.A., Khabibova N.N. Dental system
in children from mothers with gestational Arterial
hypertension // International congress on modern
education and integration. – 2020. – Vol. 5. – P. 345.
32.
Saidova L.A., Khabibova N.N. State of the
dentoalveolar system in children from mothers with
gestational hypertension // Middle Europ. Sci. Bull. –
2020. – Vol. 7. – P. 101-104.
33.
Scully C., Porte S. Oral mucosal disease:
recurrent aphthous stomatitis // Brit. J. Oral Maxillofac.
Surg. – 2008. – Vol. 46. – P. 198-206.
34.
Suter V.G.A., Sjolund S., Bornstein M.M.
Effects of Laser pain relief and wound healing of
recurrent aphthous stomatitis: a systematic review //
Laser Med. Sci. – 2017. – Vol. 32. – P. 953-963.
35.
Tappuni A.R., Kovacevic T., Shirlaw P.J.,
Challacombe S.J. Clinical assessment of disease
severity in recurrent aphthous stomatitis // J. Oral
Pathol. Med. – 2013, – Vol. 42. – P. 635-641.
36.
Tarakji B.. Baroudi K., Kharma Y. The effect
of dietary habits on the development of the recurrent
aphthous stomatitis // Niger Med. J. – 2012. – Vol. 53.
– P. 9-11.
37.
Woo S.B., Greenberg M.S. Ulcerative,
vesicular and bullous lesions. In: Greenberg MS, Glick
M, Ship JA, editors. Burket’s oral medicine; 11th ed.
– Hamilton (Canada): BC Decker, 2008. – P. 41-76.
38.
Yilman S., Cimen K.A. Familial Behçet

88
disease // Rheumatol. Int. – 2010. – Vol. 30. – P. 1107-
1109.
Аннотация.
Среди
стоматологических
заболеваний патология слизистой оболочки
полости рта занимает особое место, так как
ее возникновение и клинические проявления
часто связаны с влиянием многочисленных
местных и общих факторов. Отличительными
особенностями я афтозного стоматита у женщин
фертильного возраста, принимающих метотрексат,
были наличие выраженного болевого симптома,
вялотекущее
медленно
прогрессирующее
перманентное течение, длительный период
восстановительных процессов, увеличение и
болезненность регионарных лимфатических
узлов.
Ключевые слова:
афты, рецидив, стоматит,
язва, заболевания слизистой оболочки полости
рта, местная терапия, системная терапия.
Summary.
Among dental diseases, the pathology
of the oral mucosa has a special place, because its
occurrence and clinical manifestations are often
associated with the influence of numerous local
and general causes. The distinctive features of the
appearance of aphthous stomatitis in women of
fertile age taking methotrexate were the presence
of a pronounced pain symptom, a sluggish slowly
progressing permanent course, a prolonged period of
recovery processes, and increased and painfulness of
regional lymph nodes.
Key words:
aphtha, relapse, stomatitis, ulcer,
diseases of the oral mucosa, local therapy, systemic
therapy.
Annotatsiyasi.
Tish kasalliklari orasida og’iz
bo’shlig’i shilliq qavatining patologiyasi alohida
o’rin tutadi, chunki uning paydo bo’lishi va klinik
ko’rinishi ko’pincha ko’plab mahalliy va umumiy
omillarning ta’siri bilan bog’liq. Metotreksatni qabul
qilgan tug’ish yoshidagi ayollarda aftoz stomatitning
o’ziga xos xususiyatlari aniq og’riq belgilarining
mavjudligi, sust, asta-sekin progressiv kurs, tiklanish
jarayonlarining uzoq davom etishi, mintaqaviy limfa
tugunlarining ko’payishi va og’rig’i edi.
Kalit so’zlar:
afta, relaps, stomatit, yara, og’iz
bo’shlig’i shilliq qavati kasalliklari, mahalliy terapiya,
tizimli terapiya.
ПРОБЛЕМЫ СМЕЖНЫХ СПЕЦИАЛЬНОСТЕЙ
УДК: 616-053.2-616/.22-616.28
КЛИНИКО-НЕВРОЛОГИЧЕСКИЕ ОСОБЕННОСТИ ДЕТЕЙ С ВРОЖДЕННОЙ И
ПРИОБРЕТЕННОЙ НЕЙРОСЕНСОРНОЙ ТУГОУХОСТЬЮ
Шамансуров Ш.Ш.
1
, Махкамова Д.К.
2
, Абдукадырова И.К.
1
1
Центр развития профессиональной квалификации медицинских работников,
2
Республиканский специализированный научно-практический медицинский центр
микрохирургии глаза
По разным данным, врожденная нейросенсорная
тугоухость (НСТ) определяется в среднем у 82-
83% детей от общего числа детей с тугоухостью.
Среди причин, обусловливающих возникновение
НСТ, в 41,8% случаев были генетические
мутации, приводящие к несиндромальной (48,1%)
и синдромальной патологии слуха (13,8%). В
5,9% НСТ была обусловлена внутриутробными
инфекциями, такими как ЦМВ, герпес,
токсоплазмоз, грипп. В 5% случаев была выявлена
анте- и интранатальная гипоксия плода (5%). Среди
причин, вызвавших НСТ, были глубокая степень
недоношенности (3,1%). врожденные аномалии
развития внутреннего уха (3%), аномалии развития
наружного и среднего уха, пороки развития
челюстно-лицевого скелета (2,7%).
Несмотря на многочисленные исследования, в
которых было доказано ототоксическое влияние
антибиотиков аминогликизидного ряда, в детской
практике часто можно наблюдать неоправданное
использование этой группы препаратов у
беременных и детей младшего возраста. В 1%
случаев НСТ у новорожденных была обусловлена
ототоксичными
препаратами
во
время